
[Courtesy of EOFlow]
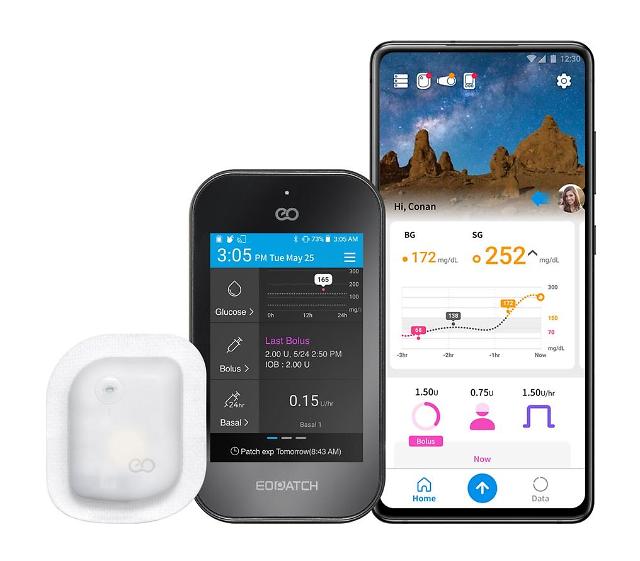
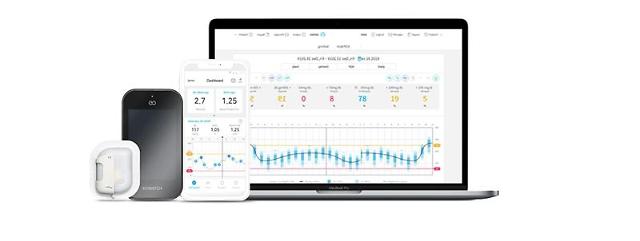
SEOUL -- EOFlow, a provider of wearable drug delivery solutions, has enrolled the first patient for a clinical study to demonstrate and validate the efficacy of EOPatch for treating people with Type 2 diabetes and expand its user base. EOPatch is the world’s second tubeless, wearable, and disposable insulin pump that enables continuous subcutaneous insulin infusion.
The clinical study involves ten leading university hospitals and up to 136 people with Type 2 diabetes. "We expect to obtain significant and meaningful trial data by around mid-2022. Based on the trial data, we intend to demonstrate and validate the safety and efficacy of therapy using EOPatch for people with type 2 diabetes, improving quality of life for them," EOFlow’s founder CEO Jesse J. Kim said in a statement on October 14.
Type 1 diabetes is a chronic condition in which the pancreas produces little or no insulin. Although type 1 diabetes usually appears during childhood or adolescence, it can develop in adults. Treatment focuses on managing blood sugar levels with insulin, diet and lifestyle to prevent complications.
Type 2 is an impairment in the way the body regulates and uses sugar as a fuel, resulting in too much sugar circulating. High blood sugar levels can lead to disorders of the circulatory, nervous and immune systems. Signs and symptoms often develop slowly, but the disease can be managed by losing weight, eating well and exercising.
EOPatch is controlled by Narsha, the world's first smartphone application that can monitor and control insulin delivery by a pump. EOFlow seeks to develop wearable drug delivery solutions in line with a trend of converting intravenous injection drugs to subcutaneous regimens that would empower users to administer drugs at home.
Copyright ⓒ Aju Press All rights reserved.


View more comments