[Courtesy of UNIST]
SEOUL -- Researchers have developed a ternary catalyst composed of cobalt, nickel and iron that can effectively convert greenhouse gases such as methane and carbon dioxide into hydrogen and carbon monoxide. The catalyst can be used for the production of renewable energy and simultaneously reduce air pollution.
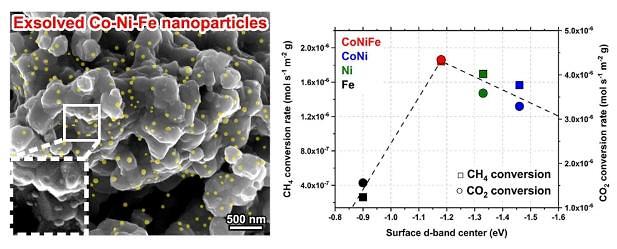
The dry reforming of methane (DRM) method produces synthetic gas (syngas), a mixture of hydrogen and carbon monoxide, through the chemical reaction between methane and carbon dioxide. Normally, the method involves a catalyst made with nickel but it gets unusable due to carbon coking, the accumulation of a hard layer of porous carbon content material, and particle agglomeration.
The Ulsan National Institute of Science and Technology (UNIST) said in a statement on June 10 that its research team has developed the ternary catalyst for the DRM production of syngas. "This research, which focused on the development of a catalyst with enhanced activity and stability, will greatly contribute to the commercialization of the DRM technique," UNIST researcher Kim Gun-tae was quoted as saying.
The catalyst's cobalt-nickel-iron alloy nanoparticles allow the generation of a larger number of exsolved nanoparticles, separated at critical temperatures, to cause more enhanced catalytic activity than the conventional catalyst's nickel monometallic and cobalt-nickel bimetallic particles.
"The activity and stability of a catalyst must be secured to stably produce syngas and hydrogen through DRM," Kim said, adding that researchers found that the ternary catalyst operated stably for more than 350 hours at a temperature of 750 degrees Celsius (1,382 Fahrenheit).




